
Some Fedida Lab Research
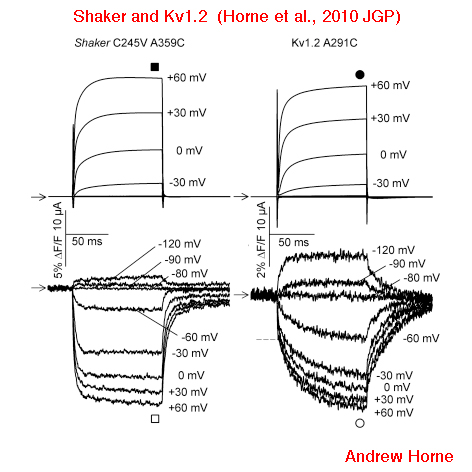
Gating Currents
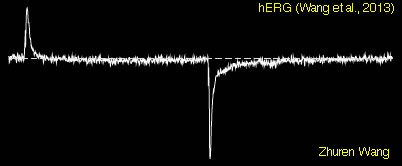
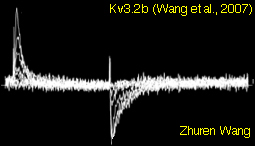

Single Channel Recordings
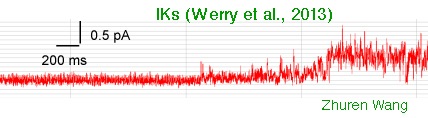
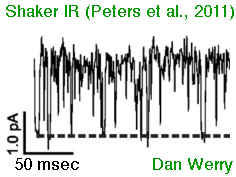

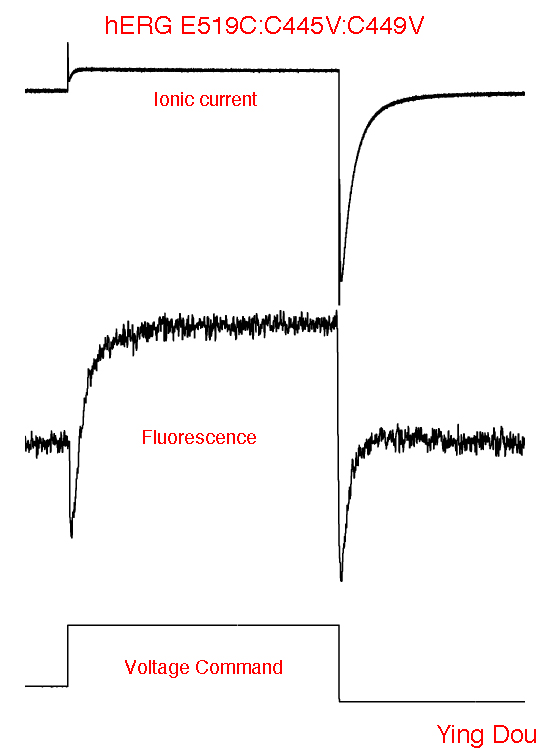
Voltage Clamp Fluorimetry
Monitoring channel rearrangement in real time
Late INa
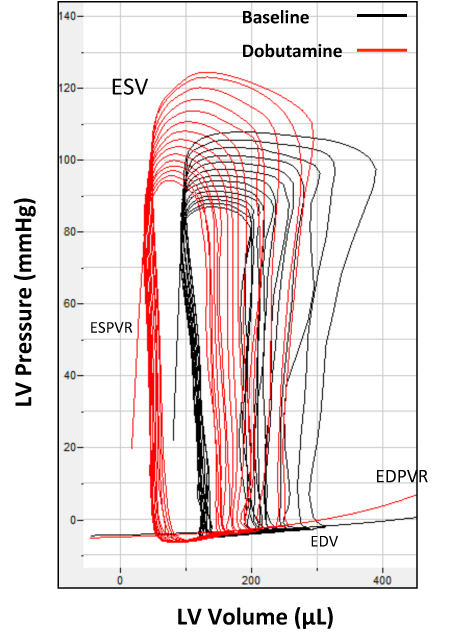
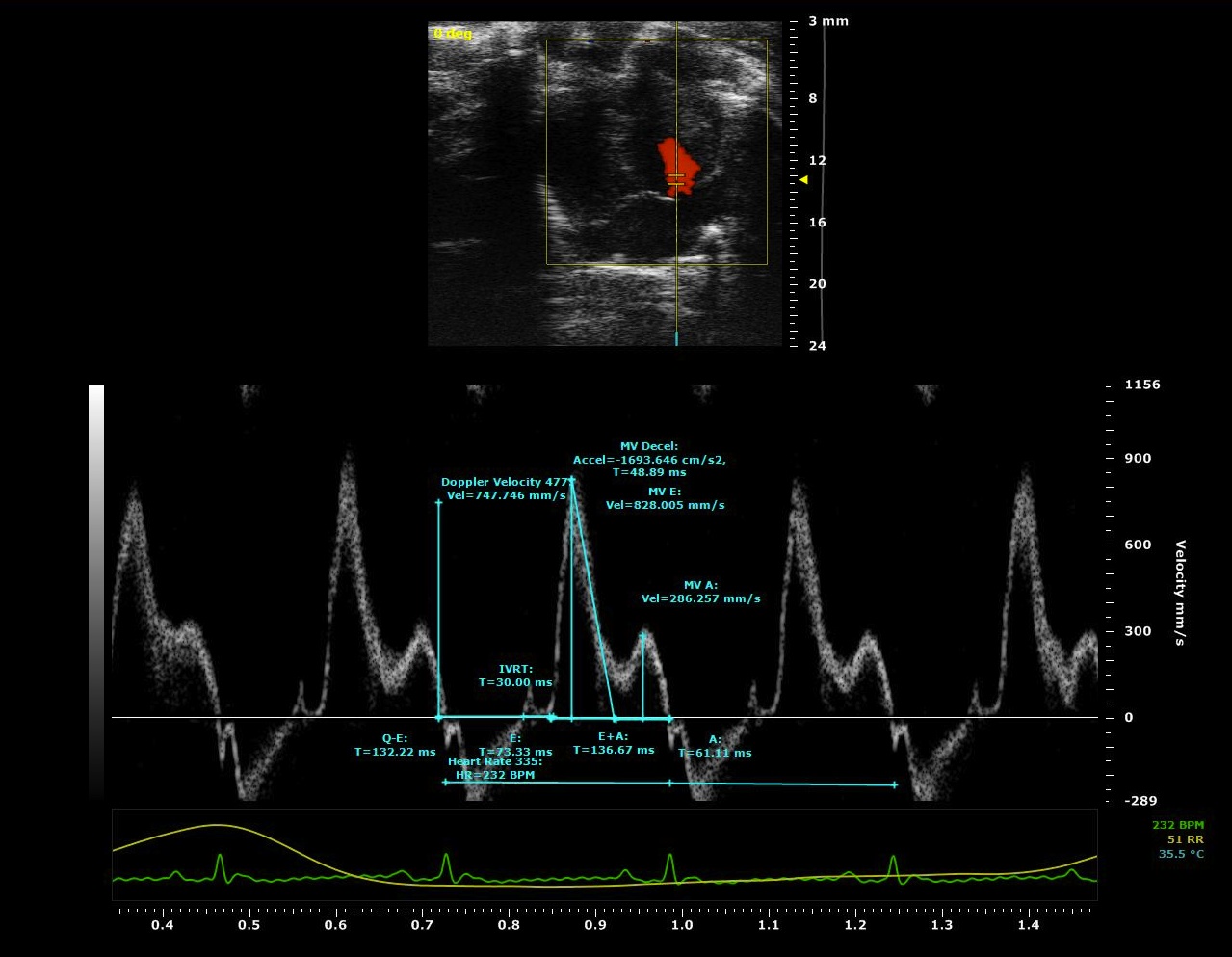
The Na channel conductance responsible for the upstroke of the action potential does not completely inactivate and a late sodium ion current (late INa) continues during the later phases of the action potential. Late INa contributes significantly to modulation of action potential morphology and accounts for at least half of the intracellular Na loading in the myocyte. Conditions in which late INa is enhanced (heart failure, hypertension, long QT syndrome) are characterized by mechanical dysfunction and arrhythmias. Cellular Ca overload occurs due to the extrusion of the extra Na via the reverse mode of the Na/Ca exchanger (NCX) or/and decreased Ca extrusion through NCX during its forward mode. Ca overload leads to increased actin/myosin filament interaction, which, during diastole increases left ventricular diastolic stiffness and results in a failure to relax normally, contributing to diastolic dysfunction. Thus, our working hypothesis is that late INa is a significant contributor to diastolic dysfunction which can result in signs and symptoms of heart failure (heart failure with preserved ejection fraction, HFpEF) and therefore an important potential therapeutic target. In order to test our hypothesis, we are determining the effects of potent, novel, specific, and selective inhibitors of late INa on diastolic function in vivo and in vitro. The experimental approach involves echocardiography measurements, pressure-volume loop analysis, whole cell patch clamp recordings, sarcomere shortening and intracellular Na/Ca measurements.
Viroporins
We are investigating the biophysical properties of viroporins, which are virally-encoded ion channels required for the life cycle of many viruses. Using whole cell expression systems and mutagenesis, we are characterizing the ion currents and sequence requirements of multiple viroporins including those from hepatitis C, influenza A, HIV-1, bovine viral diarrhea virus, and others.
Via in-house medicinal chemistry efforts and collaborators, we are also testing novel compounds for their ability to block viroporin activity. We expect that our electrophysiology-based approach will identify new mechanisms by which viroporins enhance viral life cycles, while also identifying leads for novel drugs to treat viral diseases with major impacts on human health.
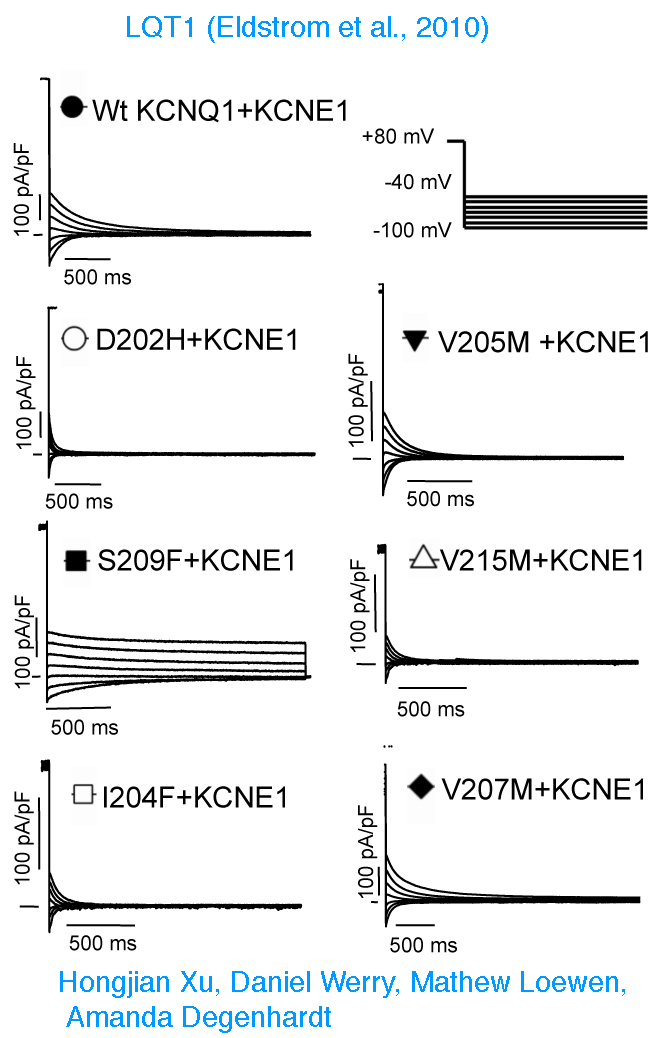
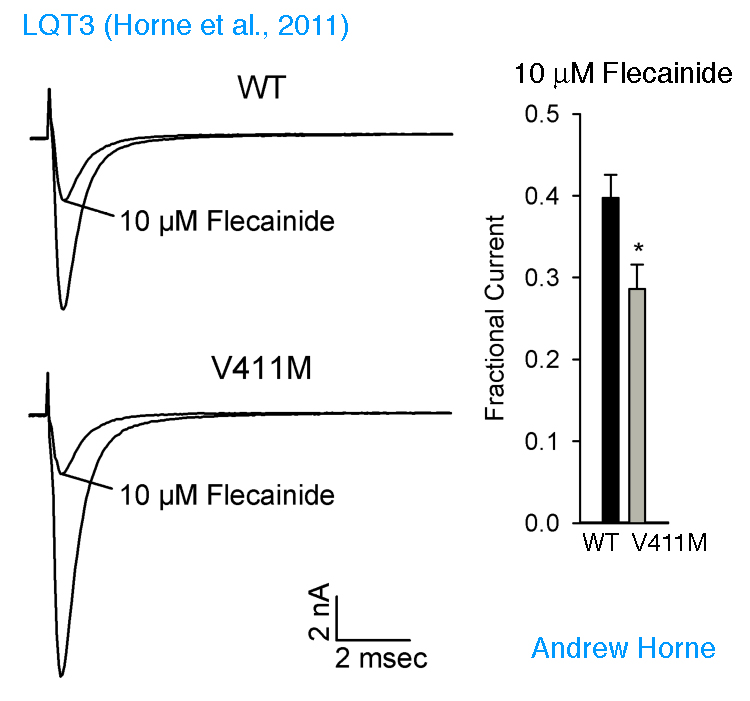
Long QT Syndrome
Ion Channel Trafficking
Our laboratory was the first to demonstrate the involvement of retrograde, microtubule-tracking dynein motor in the trafficking of a cardiac potassium channel, Kv1.5 (Choi, et al., 2005). Block of dynein function by overexpression of a dynactin subunit causes a dramatic increase in the surface expression of that channel. This is because delivery of the channel to the plasma membrane is not severely impeded but internalization from that locale is dramatically reduced. We have since implicated the dynein motor protein in the trafficking of several other potassium channels (Loewen, et al., 2009) and shown that Kinesin I is essential to the delivery of newly synthesized Kv1.5 to the cell surface (Zadeh, at al., 2009).
We have done a great deal of work delineating the compartments through which potassium channels are carried through the cell, studying the involvement of various Rab GTPases in the processes. These Rab GTPases regulate the trafficking of membrane proteins and, individually, define the various compartments through which a membrane protein may be carried. Using electrophysiological, molecular and confocal imaging techniques, we found that, upon internalization, that surface-expressed Kv1.5 is endocytosed in a Rab5-dependent manner and that a substantial fraction of the channel recycles rapidly to the plasma membrane in a Rab4-dependent process. Another fraction associates with Rab7 and traffics to the lysosome, but only a very small fraction of the channels recycles through the slower, Rabb11-dependent pathway.. (Zadeh, et al, 2008; Steele, et al., 2007).
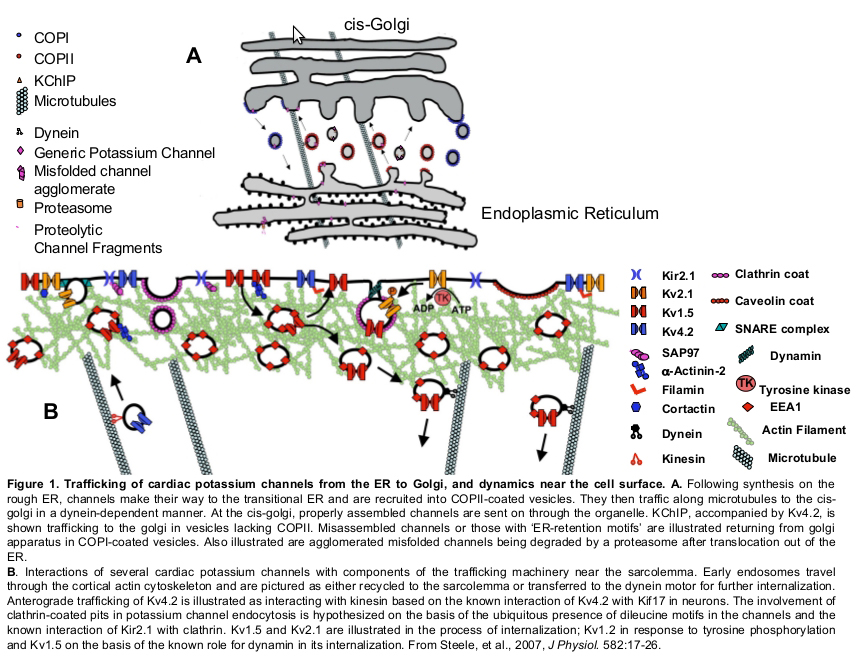
Recently, our laboratory developed the first successful methods for the transfection of adult cardiac myocytes that did not involve dedifferentiation of myocytes. Cardiomyocytes transfected by our methods retain their normal, rod-shaped, striated morphology as well as normal ionic current profiles. We have used our newly developed methods to investigate the roles of Rab GTPases and Sar1 in the trafficking of a cardiac potassium channel in its natural environment (Wang, et al., 2012). Studying endogenous Kv4.2 in this case, we found that, in the myocytes, many of the same Rab GTPases were involved in Kv4.2 trafficking as were involved in that of Kv1.5 in HEK293 cells and myoblasts. Also in the myocytes, we defined, for the first time, two of the important players in forward trafficking of a cardiac ion channel. Two GTPases, Rab 1 and Sar1, which are involved in carrying membrane proteins from the endoplasmic reticulum were found to be necessary for delivery of Kv4.2 to the plasma membrane. This was in contrast to previous reports in other cell types, where Kv4.2 trafficking has been shown to be independent of Sar1.
In collaboration with Stephane Hatem, we have also used transfected adult cardiomyocytes to in studies showing that cholesterol depletion from the plasma membrane causes a dramatic increase in Kv1.5 trafficking to the cell surface (Balse, et al., 2009). We showed this trafficking to require functional Rab11 GTPase but to be independent of new Kv1.5 synthesis.